Abstract
SL-401 is a novel targeted therapy comprised of recombinant interleukin 3 (IL3) fused to a truncated diphtheria toxin (DT) payload. SL-401 delivers DT to cells expressing the IL3 receptor (CD123). After internalization, DT catalyzes ADP ribosylation of eukaryotic elongation factor 2 (eEF2), blocking protein synthesis and killing target cells. SL-401 is currently in clinical trials for CD123+ cancers, including acute myeloid leukemia (AML) and blastic plasmacytoid dendritic cell neoplasm (BPDCN). Other than CD123 expression, the determinants of response are largely unknown. Our goal was to study mechanisms of de novo and acquired resistance to inform combination strategies and enhance the efficacy of SL-401.
In 16 AML and BPDCN patients enrolled in a phase 1-2 trial, we did not see decreased expression of CD123 by flow cytometry during or after SL-401. To study alternative resistance mechanisms, we generated 3-6 independent SL-401 resistant clones from each of 4 CD123+ AML (THP1, NOMO1, EOL1) or BPDCN (CAL1) cell lines. We treated cells with the LD95 (lethal dose to 95%), retreating upon recovery. All lines developed >2-3 log resistance to SL-401 within 28 days. All resistant clones maintained CD123 surface expression, consistent with what we observed in patients. Using confocal microscopy, we saw that a fluorescently tagged SL-401 was internalized equally in resistant and parental cells. SL-401 resistant cells were also resistant to full length DT, suggesting that the mechanism of resistance involved DT rather than IL3 binding/internalization.
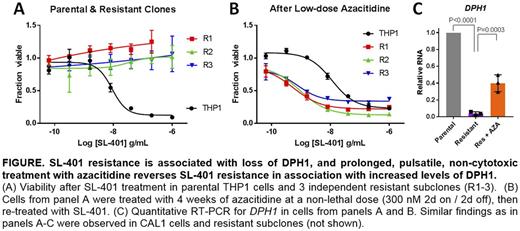
We performed whole transcriptome RNA-sequencing and whole exome sequencing (WES) on parental and SL-401 resistant cells. There were no recurrent acquired DNA mutations. However, in RNA-seq the most downregulated gene in 6 independent clones from 2 lines was DPH1 (FC -7.5, FDR<0.0001). DPH1 is the first enzyme in a cascade that converts histidine 715 on eEF2 to diphthamide, the direct target for ADP ribosylation by DT. Decreased expression of DPH1 was confirmed in the 6 resistant clones by qRT-PCR, and in 3 clones from an additional line. Across 33 cell lines and subclones there was an inverse linear correlation between DPH1 level and SL-401 IC50 (P=0.0005). To validate this finding in patients, we performed paired RNA-seq on CD45+CD123+ sorted blasts from 2 AMLs pre & post 2 cycles of SL-401. Both patients' AMLs had reduced DPH1 after exposure to SL-401 (mean -2.1 fold). To determine if loss of DPH1 was sufficient to confer de novo resistance to SL-401, we generated THP1, NOMO1, and CAL1 cells stably expressing the Cas9 nuclease and transduced them with 1 of 4 DPH1-targeting or 2 non-targeting CRISPR sgRNAs co-expressing GFP. Four days after infection at titers that achieve ~20% GFP+ cells, we treated with the LD95 of SL-401. We observed a survival advantage for DPH1 sgRNA-transduced cells (2.9 fold increase in GFP+ cells, P<0.0001) and decreased apoptosis measured by AnnexinV positivity, compared to GFP- cells in the same cultures. By contrast, there was no survival advantage for cells transduced with control sgRNAs. These data suggest that DPH1 loss is sufficient to mediate SL-401 resistance in AML and BPDCN.
Pseudomonas toxin (PT) also targets eEF2 on diphthamide, and PT resistance in a B-ALL cell line has been associated with DPH1 loss via reversible promoter CpG DNA methylation (Hu, Leuk Res 2013). Therefore, we tested the DNA methyltransferase inhibitor azacitidine in combination with SL-401 and observed synergistic cytotoxicity, in naïve (combination index (CI) = 0.45; <1 indicates synergy) and SL-401 resistant (CI = 0.55) cells. Most strikingly, 4-week pulsatile treatment with non-lethal "epigenetic" doses of azacitidine (300 nM 2d on/2d off) fully reversed SL-401 resistance in 6 CAL1 and THP1 clones that were insensitive at baseline (Figure). Controls grown in vehicle or with weekly SL-401 challenge showed no reversion, suggesting that azacitidine had a specific sensitizing effect. Restoration of SL-401 sensitivity was accompanied by an increase in DPH1 expression compared to resistant clones.
In summary, we found that DPH1 is a biomarker of SL-401 activity and acquired resistance, and resistance is reversible by azacitidine. Based on these data, we have initiated a multicenter phase 1 trial of the combination of SL-401 and azacitidine in patients with AML or MDS (NCT03113643), with correlative laboratory studies designed to explore these hypotheses.
vonEgypt: Stemline Therapeutics: Employment. Lindsay: Stemline Therapeutics: Employment. Brooks: Stemline Therapeutics: Employment, Equity Ownership, Patents & Royalties. Lane: Stemline Therapeutics: Research Funding; N-of-one: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal